Prescribing Information: ChloraPrep™ & ChloraPrep with Tint 2% chlorhexidine
gluconate w/v / 70% isopropyl alcohol v/v cutaneous solution. Refer to the Summary
of Product Characteristics before prescribing. Presentation: ChloraPrep – each
applicator contains 0.67ml, 1ml, 1.5ml, 3ml, 10.5ml or 26ml of 20 mg/ml chlorhexidine
& 0.70 ml/ml isopropyl alcohol; ChloraPrep with Tint – each applicator contains 3ml,
10.5ml or 26ml of 20 mg/ml chlorhexidine & 0.70 ml/ml isopropyl alcohol. Indication:
Disinfection of skin prior to invasive medical procedures. Dosage & administration:
Applicator volume dependent on invasive procedure being undertaken. May be
used for all age groups and patient populations. Use with care in newborn babies
and those born prematurely. Applicator squeezed to break ampoule and release
antiseptic solution onto sponge. Solution applied by gently pressing sponge against
skin and moving back and forth for 30 seconds. The area covered should be allowed
to air dry. Contra-indications: Patients with known hypersensitivity to ChloraPrep
or ChloraPrep with Tint or any of its components, especially those with a history of
possible Chlorhexidine-related allergic reactions. Warnings and precautions: Solution
is flammable. Do not use electrocautery procedures or other ignition sources until
dry. Remove any soaked materials before proceeding with the intervention. Do not
use in excessive quantities, allow to pool in patient skin folds or drip on materials in
contact with patient skin. Ensure no excess product is present prior to application
of occlusive dressing. For external use only on intact skin, do not use on open skin
wounds or broken or damaged skin. Over-vigorous use on fragile or sensitive skin or
repeated use may lead to local skin reactions. Avoid prolonged skin contact. Avoid
contact with eyes, mucous membranes, middle ear and neural tissue. Chlorhexidine
may induce hypersensitivity, including generalised allergic reactions and anaphylactic
shock. Chlorhexidine-containing products are known causes of anaphylactic reactions
during anaesthesia. The symptoms of anaphylactic reactions might be masked in an
anesthetized patient. If symptoms of an anaphylactic reaction are detected during
anaesthesia, chlorhexidine related allergic reaction should be considered. When
chlorhexidine-related allergic reaction during anaesthesia is suspected, other products
containing chlorhexidine used during anaesthesia (e.g. IV lines) should be removed.
Special precaution should be taken to avoid patient exposure to any other product
containing chlorhexidine during the course of the treatment. May cause chemical
burns in neonates, with a higher risk in preterm infants and within the first 2 weeks
of life. Pregnancy & lactation: Although no studies have been conducted, no effects
are anticipated as systemic exposure is negligible. Undesirable effects: Very rare;
allergic or irritation skin reactions to chlorhexidine, isopropyl alcohol or sunset yellow
(E110, present in ChloraPrep with Tint only), including erythema, rash, pruritus and
blisters or application site vesicles, other local symptoms have included skin burning
sensation, pain and inflammation. Frequency not known; hypersensitivity including
anaphylactic shock, dermatitis, eczema, urticaria, chemical burns in neonates, eyes
irritation, hyperaemia, impaired vision, chemical burn and eye injury. Discontinue
use at the first sign of local skin reaction. Cases of anaphylactic reactions have
been reported during anaesthesia. Description of selected adverse reactions: There
have been isolated spontaneous reports of generalised allergic reactions potentially
associated with ChloraPrep solution and have been reported during anaesthesia. In
some cases, the patient may have had a pre-existing sensitivity to chlorhexidine.
This product may cause a severe allergic reaction. Symptoms may include wheezing/
difficulty breathing, shock, facial swelling, hives, or rash. Use of ChloraPrep is contra�indicated where patients have shown previous hypersensitivity to chlorhexidine or
isopropyl alcohol (see Section Contra-indications). If hypersensitivity or an allergic
reaction occurs, stop use and seek medical help right away. Per applicator costs (ex
VAT) ChloraPrep: 0.67ml (SEPP) – UK £0.30, Ireland €0.39; 1ml – UK £0.32, Ireland
€0.41; 1.5ml (FREPP) – UK £0.55, Ireland €0.64; 1.5ml – UK £0.78, Ireland €0.94;
3ml – UK £0.85, Ireland €1.06; 10.5ml – UK £2.92, Ireland €3.79; 26ml – – UK £6.50,
Ireland €7.96. ChloraPrep with Tint: 3ml – UK £0.89, Ireland €1.09; 10.5ml – UK £3.07,
Ireland €3.88; 26ml – UK £6.83, Ireland €8.19 Legal category: UK: GSL. Ireland: Not
subject to medical prescription. Marketing Authorisation Numbers: ChloraPrep,
(UK: PL05920/0002-001; Ireland: PA2287/001/002) & ChloraPrep with Tint, (UK:
PL05920/0003-0001; Ireland: PA2287/001/001) Marketing Authorisation Holder:
UK : Becton Dickinson UK Ltd, 1030 Eskdale Road, Winnersh, Wokingham RG41 5TS,
United Kingdom. Ireland : Becton Dickinson France, 11 Rue Aristide Bergès, 38800 Le
Pont de Claix, France Date of Revision of the API: April 2021.
Reporting suspected adverse reactions is important to monitor the benefit/risk
balance of the medicinal product. Reporting forms and information can be found
at www.mhra.gov.uk/yellowcard (for UK) and www.hpra.ie (for Ireland). Adverse
events should also be reported to BD Freephone number: For UK 0800 0437 546
or email: SafetyInformation@bd.com. For Ireland: +353 01 4287895/7896 or email:
References
1. Silva P. J Perioper Pract 2013; 23(4): 87-90.
2. McGrath DR, McCrory D. Ann R Coll Surg Engl 2005; 87(5):366-8.
3. El-Othmani MM et al. Int Surg J 2016; 3(1): 1-10.
4. MHRA. Public Assessment Report 2012: UK/H/1305/02/DC.
4.1. Gahlot R et al. Int J Crit Illn Inj Sci 2014; 4(2): 162-7.
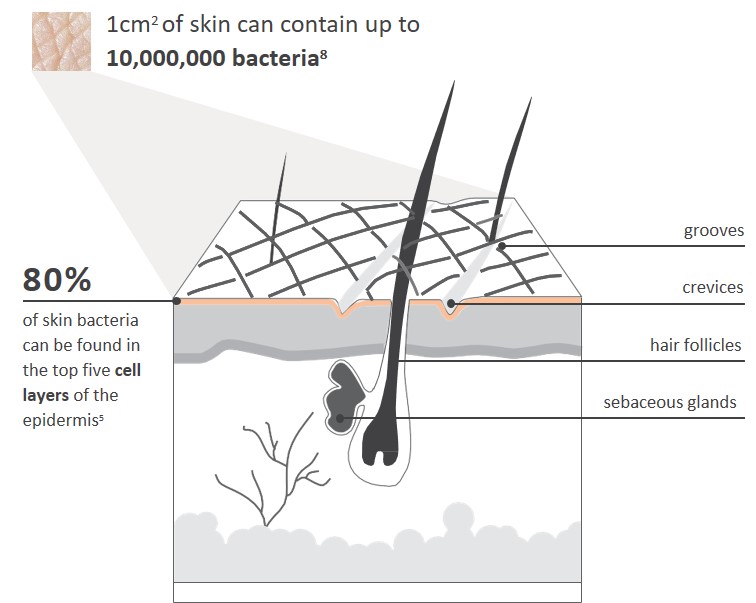
5. Inwood S. Br J Nurs 2007; 16(22): 1390-4.
7. Moureau NL. Outpatient Surgery Online 2010; May: 31-3.
8. Fredricks DN. J Invest Dermatol Symp Proc 2001; 6(3): 167-9.